electron affinity of sodium|A7: Electron Affinities : Tagatay Electron affinity can be defined in two equivalent ways. First, as the energy that is released by adding an electron to an isolated gaseous atom. The second (reverse) definition is that electron affinity is the energy required to remove an electron from a singly charged gaseous negative ion. The latter can be regarded as the ionization energy of the –1 ion or the zeroth ionization energy. Either convention can be used. 🏴 Premier League Matchweek 2 takes center stage this weekend! ⚽🔥 Who are you backing? Make your predictions now on SportyBet! 👇 sporty.bet/Engla. GetSporty #BetSporty #PremierLeague
electron affinity of sodium,Ago 11, 2023
Electron affinity is defined as the change in energy (in kJ/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom to form a negative .Electron affinity can be defined in two equivalent ways. First, as the energy that is released by adding an electron to an isolated gaseous atom. The second (reverse) definition is that electron affinity is the energy required to remove an electron from a singly charged gaseous negative ion. The latter can be regarded as the ionization energy of the –1 ion or the zeroth ionization energy. Either convention can be used.

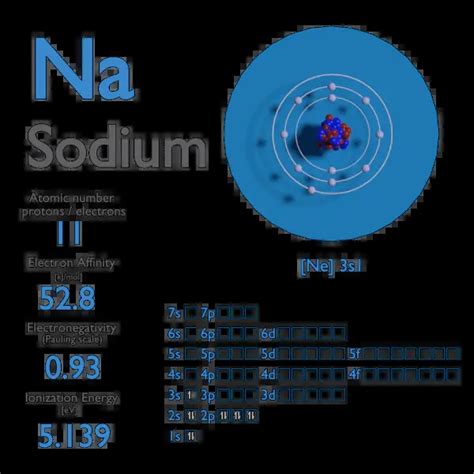
Electron affinity of Sodium is 52.8 kJ/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: the change in energy (in .
The electron affinity of an element is the energy given off when a neutral atom in the gas phase gains an extra electron to form a negatively charged ion. A fluorine atom in the gas phase, for example, gives off energy .Use the trends in electron affinities going down a column for elements in the same group. Similarly, use the trends in electron affinities from left to right for elements in the same .A representation of the atomic spectrum of sodium. Ionisation Energies and electron affinity. The electron affinity of sodium is 52.8 kJ mol ‑1. The ionisation energies of sodium are given below.electron affinity of sodium A7: Electron Affinities The electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or molecule in the gaseous state to form a negative ion. X(g) .The amount of energy released when an electron is added to a neutral atom to form an anion is called electron affinity. Electron affinities are difficult to measure. Electron affinity increases going left to right across .The electron affinity ( Eea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state .Sodium (Na): 53 KJ mol-1; Potassium (K): 48 KJ mol-1; . Electron affinity increases upward for the groups and from left to right across periods of a periodic table because the electrons added to energy levels become closer to the nucleus, thus a stronger attraction between the nucleus and its electrons. Remember that greater the distance, the .electron affinity of sodiumElectron Affinity. The electron affinity (EA) of an element E is defined as the energy change that occurs when an electron is added to a gaseous atom or ion: [latex]E_{(g)}+e^- \rightarrow E^-_{(g)} \;\;\; \text{energy . Definition of Electron Affinity. Electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral gaseous atom. The more negative the . Electron affinity of Lithium, Sodium, Potassium, Rubidium . Element: Electron affinity(KJ/mol-1) Li-59.8: Na-53: K-48.9: Rb-46.9: Variation along a period. The atomic size reduces as we move through a period, but the nuclear charge increases. The combined effect of these two causes increases the force of attraction for the electron, so .
Electron Affinities. Electron affinity, often abbreviated as EA, is the energy released when an electron is added to a valence shell of the atom. F(g) + e - -> F-(g) EA = -328 kJ/mol [When an electron is added to an atom, energy is .
The -349 is the first electron affinity of chlorine. Remember that first electron affinities go from gaseous atoms to gaseous singly charged negative ions. And finally, we have the positive and negative gaseous ions that we can convert into the solid sodium chloride using the lattice formation enthalpy.
Calculated Electron Affinities Caution! Results of electron affinity calculations are very dependent on the basis set. Many species have anions that are not bound with respect to a free electron and the neutral species, in which case the calculations may give results that are not meaningful.
The electron affinity of sodium is lower than that of lithium, while the electron affinity of chlorine is higher than that of fluorine. Suggest an explanation for this observation. Verified Solution. This video solution was recommended by our tutors as helpful for the problem above. Video duration: 3m. Play a video:No headers. The electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or molecule in the gaseous state to form a negative ion \[X_{(g)} + e^− \rightarrow X^−_{(g)} + energy\] Calcium has two electrons in its outermost shell. So it will tend to lose those electrons not to gain one extra electron. This means electron affinity of calcium is less than that of fluorine. So fluorine is definitely not the answer (as we have to find an element with the least electron affinity). Sodium and potassium both have one electron in .A7: Electron Affinities Ionisation Energies and electron affinity. The electron affinity of sodium is 52.8 kJ mol ‑1. . Electron binding energies for sodium. All values of electron binding energies are given in eV. The binding energies are .
Electron Affinity – Sodium. Electron affinity of Sodium is 52.8 kJ/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: the change in energy (in kJ/mole) of a neutral atom or .The electron affinity in a semiconductor is defined as the energy difference between the bottom of the conduction band and the “vacuum level” (the minimum energy of an electron in vacuum). From: Characterization of Semiconductor Heterostructures and Nanostructures, 2008. About this page.
A wave function of the form Φ = ϕ (r 1, r 2) (1 + c r 12) is assumed for the (3 s) electrons of Na −, to take into account the polarization effect of the added electron, an effect neglected by the Hartree method.On this basis the electron affinity of sodium is calculated to be +1.2 ev and therefore the negative ion is stable.
In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: the change in energy (in kJ/mole) of a neutral atom or molecule when an electron is added to the atom to form a negative ion. . Sodium is a chemical element with atomic number . In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: the change in energy (in kJ/mole) of a neutral atom or molecule when an electron is added to the atom to form a negative ion. . Sodium is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic .Explore Electron affinity explainer video from Chemistry 101 on Numerade. . And so, going into our last example, let's say that you want to rank the electron affinity for sodium rubidium, potassium and hydrogen, so sodium is located here. Hydrogen is here. Calcium here and we bring them is here. And so we know that going down a period, you .
The height corresponds to the measured value of the electron affinity of the corresponding element 7,8,67.Astatine is highlighted in red. Blue indicates elements that are experimentally determined .
electron affinity of sodium|A7: Electron Affinities
PH0 · What is Electron Affinity?
PH1 · WebElements Periodic Table » Sodium » properties of free atoms
PH2 · WebElements Periodic Table » Sodium » properties of
PH3 · Sodium
PH4 · Ionization Energy and Electron Affinity
PH5 · Electron affinity (data page)
PH6 · Electron affinity
PH7 · Electron Affinity Chart (Labeled Periodic table + List)
PH8 · Electron Affinity
PH9 · A7: Electron Affinities
PH10 · 7.5: Electron Affinities